Multicenter Clinical Trial Management
UCLA Brain Tumor Imaging Laboratory (BTIL) provides comprehensive multicenter clinical trial management services to ensure consistency, reliability, and quality across diverse medical centers. Streamlining data acquisition and harmonizing imaging protocols can guarantee consistent and dependable results for multicenter clinical trials. Join us in advancing brain tumor diagnostics and therapeutics on a broader scale.
Explore our services
Image Protocol
Standardization
Data
Management
Quality
Assurance
Image Analysis
& RANO Reads
Reporting
Selected References
1. |
Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, Nelson SJ, Gerstner E, Alexander B, Goldmacher G, Wick W, Vogelbaum M, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Pope WB, Yang D, Chung C, Knopp MV, Cha S, van den Bent MJ, Chang S, Yung WK, Cloughesy TF, Wen PY, Gilbert MR; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee. |
2. |
Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: guideline statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Barajas RF, Politi LS, Anzalone N, Schöder H, Fox CP, Boxerman JL, Kaufmann TJ, Quarles CC, Ellingson BM, Auer D, Andronesi OC, Ferreri AJM, Mrugala MM, Grommes C, Neuwelt EA, Ambady P, Rubenstein JL, Illerhaus G, Nagane M, Batchelor TT, Hu LS. |
3. |
Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Ellingson BM, Wen PY, Cloughesy TF. |
4. |
The Future Glioblastoma Clinical Trials Landscape: Early Phase 0, Window of Opportunity, and Adaptive Phase I-III Studies. Cho NS, Wong WK, Nghiemphu PL, Cloughesy TF, Ellingson BM. |
5. |
Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas Boxerman JL, Quarles CC, Hu LS, Erickson BJ, Gerstner ER, Smits M, Kaufmann TJ, Barboriak DP, Huang RH, Wick W, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Chung C, van den Bent MJ, Chang S, Al Yung WK, Cloughesy TF, Wen PY, Gilbert MR, Rosen BR, Ellingson BM, Schmainda KM; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee. |
6. |
Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases Kaufmann TJ, Smits M, Boxerman J, Huang R, Barboriak DP, Weller M, Chung C, Tsien C, Brown PD, Shankar L, Galanis E, Gerstner E, van den Bent MJ, Burns TC, Parney IF, Dunn G, Brastianos PK, Lin NU, Wen PY, Ellingson BM. Neuro Oncol. 2020 Jun 9;22(6):757-772. doi: 10.1093/neuonc/noaa030. |
7. |
National Cancer Institute Collaborative Workshop on Shaping the Landscape of Brain Metastases Research: challenges and recommended priorities Kim MM, Mehta MP, Smart DK, Steeg PS, Hong JA, Espey MG, Prasanna PG, Crandon L, Hodgdon C, Kozak N, Armstrong TS, Morikawa A, Willmarth N, Tanner K, Boire A, Gephart MH, Margolin KA, Hattangadi-Gluth J, Tawbi H, Trifiletti DM, Chung C, Basu-Roy U, Burns R, Oliva ICG, Aizer AA, Anders CK, Davis J, Ahluwalia MS, Chiang V, Li J, Kotecha R, Formenti SC, Ellingson BM, Gondi V, Sperduto PW, Barnholtz-Sloan JS, Rodon J, Lee EQ, Khasraw M, Yeboa DN, Brastianos PK, Galanis E, Coleman CN, Ahmed MM. Lancet Oncol. 2023 Aug;24(8):e344-e354. doi: 10.1016/S1470-2045(23)00297-8. PMID: 37541280. |
8. |
Radiographic read paradigms and the roles of the central imaging laboratory in neuro-oncology clinical trials. Ellingson BM, Brown MS, Boxerman JL, Gerstner ER, Kaufmann TJ, Cole PE, Bacha JA, Leung D, Barone A, Colman H, van den Bent MJ, Wen PY, Alfred Yung WK, Cloughesy TF, Goldin JG. Neuro Oncol. 2021 Feb 25;23(2):189-198. doi: 10.1093/neuonc/noaa253. PMID: 33130879; |
9. |
Radiographic Response Assessment Strategies for Early-Phase Brain Trials in Complex Tumor Types and Drug Combinations: from Digital "Flipbooks" to Control Systems Theory. Neurotherapeutics. Ellingson BM, Levin VA, Cloughesy TF. 2022 Oct;19(6):1855-1868. doi: 10.1007/s13311-022-01241-8. Epub 2022 Apr 22. PMID: 35451676; PMCID: PMC9723080. |
10. |
Therapeutic Response Assessment of High-Grade Gliomas During Early-Phase Drug Development in the Era of Molecular and Immunotherapies. Ellingson BM, Wen PY, Cloughesy TF. Cancer J. 2021 Sep-Oct 01;27(5):395-403. doi: 10.1097/PPO.0000000000000543. PMID: 34570454; PMCID: PMC8480435. |
11. |
Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between objective response rate and median overall survival. Ellingson BM, Wen PY, Chang SM, van den Bent M, Vogelbaum MA, Li G, Li S, Kim J, Youssef G, Wick W, Lassman AB, Gilbert MR, de Groot JF, Weller M, Galanis E, Cloughesy TF. Neuro Oncol. 2023 Jun 2;25(6):1017-1028. doi: 10.1093/neuonc/noad002. PMID: 36617262; PMCID: PMC10237425. |
For more information, please contact: btil@mednet.ucla.edu
UCLA Clinical Trials
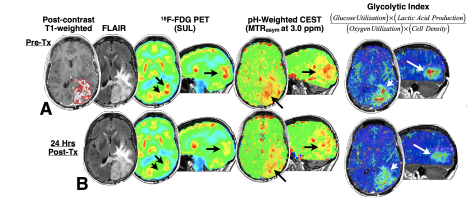
Biologic Association Between Metabolic MR-PET and Tissue Measures of Glycolysis in Brain Tumors
IRB#: 21-002007
If you have been diagnosed with a type of brain cancer called Glioblastoma and are scheduled to undergo a surgical procedure with biopsy to remove the tumor, then you may be eligible to participate in a research study to test whether a combined magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging technique is useful in identifying areas of tumor that are particularly active or aggressive.
If you choose to participate, you will have one PET scan and a 15-minute research MRI in addition to your standard pre-surgical MRI. The study will pay for research-related items and/or services.
Simultaneous Multinuclear (NA+/H+) Metabolic MRI in Brain Tumors
IRB#: 21-000514
Surgical Group
If you have a brain tumor and are candidate for surgery, you may be eligible to participate in a research study where sodium, pH and oxygen imaging are used in conjunction to identify areas of abnormal metabolism.
If you choose to participate, you will receive one MRI before surgery that will last upto 90 minutes, 60 minutes of the clinically indicated pre-surgical MRI and 30 additional minutes for the research sequences. The study will pay for the 30-minute research scan.
Immunotherapy Group
If you have been diagnosed with a recurrent high-grade glioma and your physician is planning to initiate treatment with immunotherapy, then you are eligible to participate in a research study where we explore if sodium +pH imaging can be used to predict or quantify response to immunotherapies.
If you choose to participate, you will receive two MRI scans. The first scan will last up to 90 minutes and be completed before your immunotherapy treatment and is entirely covered by the grant. The second scan will be completed following your first immunotherapy treatment and will last up to 90 minutes. The study will pay for the additional 30-minute research scan.
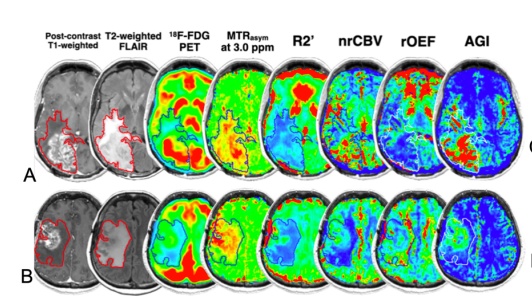
Exploration into the Association between DCN expression and MR phenotypes in GBM
IRB#: 21-002112
If you have a suspected Glioblastoma and you are candidate for a tumor resection surgery, then you may be eligible to participate in an imaging research study which observes different MR phenotypes and corresponding imaging biomarkers to understand how different tumors respond to anti-VEGF therapies. The study also aims to show an association between Decroin expression and favorable MR characteristics.
If you choose to participate, you will receive one MRI scan before surgery which will contain the special sequences and you will have 5ml of blood plasma specimen drawn for analysis of Decorin. Research Scans will be paid for by the study. If your clinically indicated scan was done before enrollment, we may only collect data from the clinical scan. No additional research time may be added to standard of care scans done prior to enrollment.
Surgical Validation of Advanced MRI Techniques in Suspected Brain Tumors
IRB#: 14-001261
If you are ≥ 18 years old and have been diagnosed with a primary brain tumor and are a candidate for surgery you may be eligible to participate in a research study to collect information from your Brain MRI exam to help improve early detection and understand brain tumor metabolism.
If you choose to participate, you will have a 60 minute Brain MRI as part of a routine pre-surgical workup plus an additional research MRI for up to 15 extra minutes. There is no fee to participate in the study. The study will pay for the entire 75 minutes MRI exam.

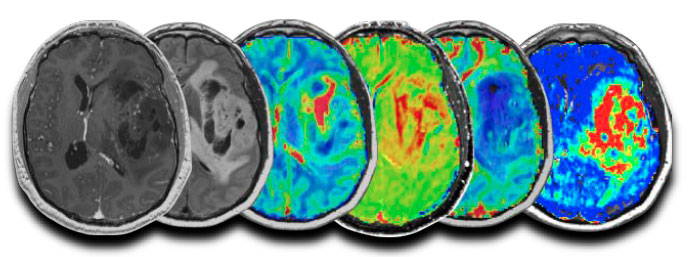
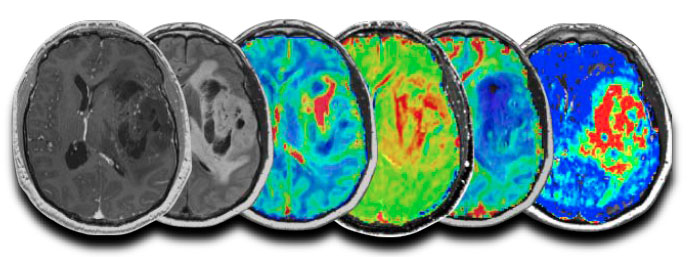
Validation Of Advanced Mri In Brain Tumors Treated With Immunotherapies Or Bevacizumab
IRB#: 15-000615
If you are ≥ 18 years old and you have been diagnosed with primary or recurrent high-grade gliomas, you may be eligible to participate in a research study to collect information from you Brain MRI exams to help improve early detection and understand brain tumor metabolism.
If you choose to participate, you will have 60 minutes pre- and post-treatment Brain MRI scans as part of a routine workup plus additional research MRI scans for up to 15 extra minutes.
There is no fee to participate in the study. The study will pay for the entire 75 minutes MRI exam or the additional 15 minutes of research scans.